- Research
- Open access
- Published:
The prognostic value of triglyceride-glucose index to adverse renal outcomes in patients with type 2 diabetes mellitus: results from the cohort study of ACCORD
Diabetology & Metabolic Syndrome volume 16, Article number: 201 (2024)
Abstract
Background
The triglyceride-glucose (TyG) index is a new and good biomarker of insulin resistance (IR). The prognostic utility of the TyG index for patients with type 2 diabetes mellitus (T2DM) remains uncertain. Our study seeks to elucidate the connection between the TyG index and adverse renal outcomes within a T2DM population, while also examining if these relationships are influenced by subgroup variations.
Methods
We analyzed data from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, involving 10,196 T2DM participants, to assess the link between triglyceride-glucose levels and adverse renal outcomes. This evaluation included Restricted Cubic Spline (RCS) analysis, Kaplan–Meier survival analysis, and Multivariate Cox proportional regression. Additionally, we examined the interaction between subgroups concerning adverse renal outcomes.
Results
During a 7-year follow-up, 5824 patients (57.1%) experienced worsening renal function, 2309 patients (23.2%) developed albuminuria, and 280 patients (2.7%) advanced to renal failure. After adjusting for a range of confounding variables, triglyceride-glucose levels were significantly linked to both worsening renal function (p < 0.001) and the onset of albuminuria (p = 0.020). Nonetheless, no significant association was observed between triglyceride-glucose levels and renal failure (p = 0.247). Furthermore, there was no significant subgroups interaction to the associations between TyG levels and adverse renal outcomes.
Conclusion
Our study underscores the significant relationship between the triglyceride-glucose index and the risk of adverse renal outcomes in patients with T2DM. The TyG index, as a readily calculable measure, offers clinicians a valuable tool for anticipating the risk of adverse renal outcomes in this patient population.
Introduction
The global prevalence of diabetes among adults aged 20 to 79 years is projected to rise from 6.4%, affecting 285 million individuals in 2010, to 7.7%, impacting 439 million adults by 2030. This period is expected to witness a 69% surge in the number of adults with diabetes in developing countries and a 20% increase in developed countries [1]. The kidney represents a critical site of microvascular damage in diabetes, with approximately 50% of individuals with type 2 diabetes mellitus (T2DM) developing diabetic kidney disease (DKD), characterized by impaired renal function, elevated urinary albumin excretion, or both [2]. DKD has emerged as the leading cause of end-stage renal disease (ESRD) in the United States and many developed nations, accounting for 30–50% of new ESRD cases [3]. Among the long-term complications of diabetes, DKD places the most substantial burden on patients, manifesting in significant financial costs and adverse impacts on daily life. Notably, individuals with DKD face a heightened risk of adverse health outcomes, such as frailty, reduced quality of life, ESRD, progressive damage to other organs, and premature death. The majority of excess mortality associated with T2DM is notably concentrated among those suffering from DKD [4], underscoring the imperative to mitigate the incidence of adverse renal outcomes and alleviate the associated prognosis through early diagnosis and intervention.
Insulin resistance (IR), a state wherein physiological concentrations of insulin elicit a diminished biological response, has been implicated in the pathogenesis of various metabolic disorders [5]. The triglyceride-glucose (TyG) index has been developed as a biochemical surrogate for the identification of IR in both diabetic and nondiabetic individuals [6]. While the hyper-insulinemic-euglycemic clamp test remains the gold standard for assessing IR, this method is time-consuming and laborious which renders it impractical for widespread clinical application [7]. Consequently, the TyG index, derived from fasting triglyceride and glucose levels, has gained recognition as a straightforward, accessible, and cost-effective surrogate marker for IR [8]. Previous research has established the TyG index as an independent predictor of future stroke, myocardial infarction, cardiovascular mortality, and all-cause/non-cardiovascular mortality in the general population, highlighting its role in forecasting cardiovascular and metabolic diseases [9,10,11]. Moreover, the TyG index has demonstrated clinical utility in predicting adverse cardiovascular events in patients with or without diabetes who have pre-existing cardiovascular disease [12,13,14]. However, the association between TyG and the risk of adverse renal outcomes remains less well-defined. While several studies have reported a correlation between elevated TyG levels and an increased risk of chronic kidney disease (CKD) [15,16,17,18], others, such as Pan et al., have not identified a significant association between TyG levels and CKD in patients with T2DM [19]. The inconsistency in these findings can be attributed to limitations in sample sizes, differences in study populations, and varying degrees of adjustment for confounding factors. As such, further research with specific populations, larger sample sizes and adjusting for a range of confounding variables is warranted to clarify the relationship between TyG and CKD.
Therefore, this study aims to assess the associations between the TyG index and adverse renal outcomes in patients with T2DM utilizing data from the ACCORD trial and to explore potential modifications of these associations within subgroups.
Methods
Study design and participants
Our study engaged in a retrospective analysis utilizing data derived from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, funded by the National Heart, Lung, and Blood Institute. The ACCORD trial was characterized by a multicenter, randomized, double 2 × 2 factorial design, aimed at exploring the effects of three distinct medical treatment strategies on the morbidity and mortality among individuals with T2DM. These strategies included the glycemia, lipid, and blood pressure trials, each designed to evaluate their respective impacts on cardiovascular disease (CVD) outcomes. The trial enrolled 10,251 middle-aged and elderly participants, diagnosed with T2DM, featuring an average glycated hemoglobin level of 8.3% and a median diabetes duration of 10 years. Recruitment spanned from June 2001 to October 2005, across 77 research sites in the United States and Canada. Inclusion criteria targeted individuals at high risk for CVD events, attributable to either existing clinical CVD, a pronounced likelihood of CVD, or the presence of two or more CVD high-risk factors. The specific criteria for inclusion and exclusion were detailed in the foundational ACCORD study documentation [20]. Our analysis excluded participants who lacked baseline TyG values. Notably, the employment of the ACCORD dataset in our investigation received approval from the National Heart, Lung, and Blood Institute, thus upholding the required ethical and regulatory standards.
Data collection and outcomes
The dataset for this analysis comprised a comprehensive array of variables, including demographic details (age, sex, race, educational attainment, body mass index, smoking status, and alcohol use). Clinical indicators common to the cohort were also meticulously recorded, encompassing blood pressure, glycated hemoglobin (HbA1c) levels, the duration of diabetes, cardiovascular disease history, lipid profiles, heart rate, and details of treatment regimens. Additionally, established kidney risk factors, such as estimated glomerular filtration rate (eGFR), serum creatinine (SCr) levels, and urinary albumin levels, were evaluated. The TyG index, serving as a key variable, was calculated using the formula: TyG index = Ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL) / 2].
Initially, the cohort consisted of 10,251 individuals diagnosed with type 2 diabetes mellitus, from which those lacking baseline TyG values were excluded, narrowing the focus to 10,196 patients for our study.
The study specifically aimed to explore three adverse renal outcomes based on ACCORD trial definitions: the doubling of serum creatinine levels or a significant decrease in estimated glomerular filtration rate by more than 20 mL/min corresponding to worsening renal function, the onset of albuminuria, and the occurrence of renal failure including end-stage renal disease or serum creatinine levels exceeding 3.3 mg/dL corresponding.
Statistical analysis
In our analysis, continuous variables were summarized using either the mean and standard deviation or the median and interquartile range, while categorical variables were expressed as proportions. To compare groups, we applied the unpaired Student’s t test or the Mann–Whitney U test for continuous variables and the Chi-square or Fisher’s exact test for categorical variables. The cutoff point for TyG levels was determined through maximally selected log-rank statistics. Kaplan–Meier Survival analysis and Cox proportional hazards models, adjusted for potential confounders, were utilized to calculate the prevalence of events and estimate survival times across TyG categories, and to analyze the time-to-event outcomes, respectively. To explore the relationship between TyG levels and various adverse renal outcomes, we employed restricted cubic spline analysis, allowing for the investigation of both linear and nonlinear associations. Model adjustments were made in three stages, based on established potential confounders of TyG’s association with renal outcomes. The model 1 adjusted for demographics and clinical measures including age, sex, education attainment, race, smoking status, alcohol use, blood pressures, and body mass index (BMI). The model 2 added metabolic factors such as glycated hemoglobin (HbA1c), duration of diabetes, serum creatinine, and urinary albumin to the adjustments. Finally, model 3 further adjusted for the use of angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB) treatment, glycemia trial, blood pressure trial, and lipid trial. All analyses were conducted using R version 4.3.0. A two-sided p value<0.05 was considered statistically significant in our analysis.
Results
Baseline characteristics of the patients
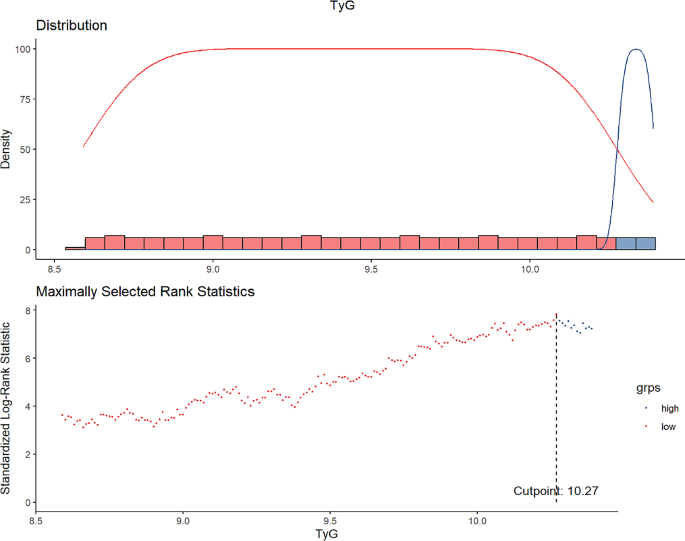
The baseline characteristics of the study cohort were comprehensively outlined in Table 1. With an average age of 62.77 years, the demographic distribution included 61.5% males and 62.5% identifying as White. The median TyG index was established at 9.10. Notably, a critical threshold for serum TyG levels linked to adverse renal outcomes was identified as 10.27, determined through maximally selected log-rank statistics, depicted in Fig. 1. A detailed analysis of baseline demographic and biochemical measurements, categorized by TyG levels, was presented in Table 1. This analysis underscored the association of elevated baseline TyG levels with several demographic and clinical parameters, including age, sex, race, educational attainment, BMI, duration of diabetes, SBP, DBP, heart rate, the use of insulin, HbA1c, FPG, and lipid profiles (TC, TG, HDL-C, VLDL-C), as well as serum potassium, estimated glomerular filtration rate, serum creatinine, urinary creatinine, urinary albumin and urinary albumin to creatinine ratio (UACR) levels. Higher TyG levels correlated with increased triglycerides, BMI, HbA1c, and fasting plasma glucose, indicating more severe insulin resistance. Moreover, patients with elevated TyG levels demonstrated a significantly higher risk of developing adverse renal outcomes, as statistically substantiated by the p value for worsening renal function (p < 0.001), albuminuria (p = 0.003) and renal failure (p = 0.045), as shown in Table 1.
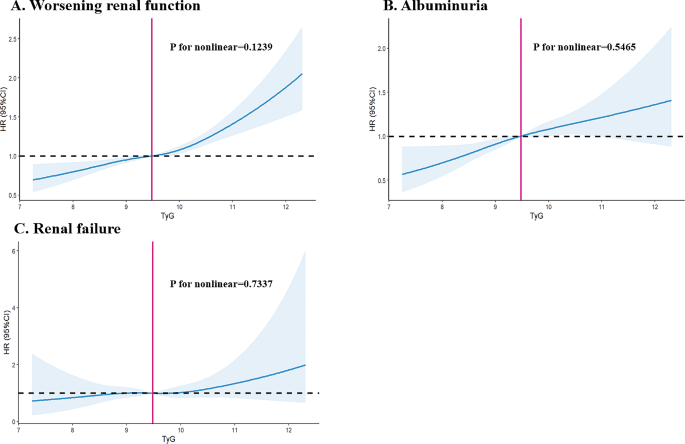
The linear or nonlinear relationship between TyG and adverse renal outcomes
In our investigation, we explored the nature of the relationship—whether linear or nonlinear—between the TyG index and various adverse renal outcomes. Through the application of restricted cubic spline analysis, we sought to thoroughly examine this association. The analysis yielded evidence of a linear relationship between TyG levels and the adverse renal outcomes, namely worsening renal function (p for nonlinearity = 0.1239), albuminuria (p for nonlinearity = 0.5465), and renal failure (p for nonlinearity = 0.7337). Consequently, these results suggested collectively that the risk associated with adverse renal outcomes increases in a linear manner in relation to TyG levels.
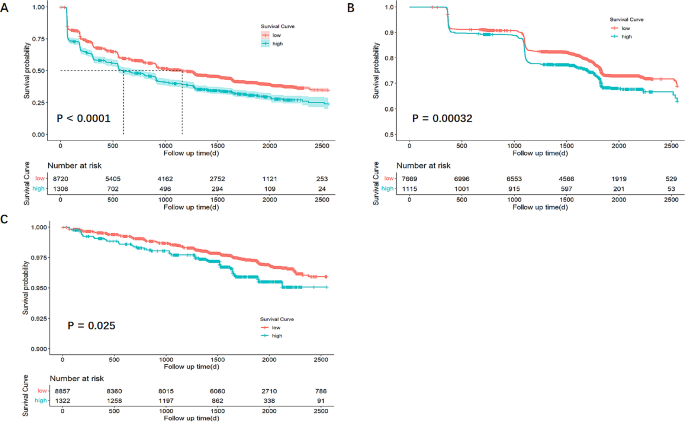
The risk of adverse renal outcomes were related with TyG levels
The association between TyG levels and the risks of adverse renal outcomes was elucidated through Kaplan-Meier survival analysis, with the findings presented in Fig. 3. This analysis revealed that individuals with lower TyG levels exhibited a higher survival probability, implying a more favorable renal prognosis. The log-rank test was utilized to evaluate the statistical significance of the observed differences, indicating that higher TyG levels were significantly associated with an increased risk of adverse renal outcomes. Specifically, the disparities for worsening renal function, albuminuria, and renal failure were statistically significant, with p value of less than 0.0001 for worsening renal function, 0.00032 for albuminuria, and 0.025 for renal failure, underscoring the critical impact of TyG levels on renal health.
Baseline TyG levels and adverse renal outcomes
In evaluating the relationship between baseline TyG levels and adverse renal outcomes, our study implemented Cox proportional regression analysis across three progressively adjusted models. Model 1 accounted for age, sex, education attainment, race, BMI, smoking status, alcohol use, DBP, and SBP. Model 2 expanded upon Model 1 by including HbA1c, diabetes duration, SCr and UACR. Model 3 further incorporated treatments with ACEI, ARB, glycemia trial, blood pressure trial, and lipid trial, building on the covariates of Model 2. The analyses demonstrated that, following adjustments for both demographic and conventional renal risk factors in Model 3, baseline TyG levels were independently associated with the incidence of adverse renal outcomes in patients with T2DM. Specifically, the hazard ratios (HRs) for the adverse renal outcomes in comparison to lower TyG levels were as follows: For worsening renal function, HR was 1.24 (95% CI 1.14–1.35, p < 0.001); for albuminuria, HR was 1.19 (95% CI 1.03–1.37, p = 0.020); and for renal failure, HR was 1.24 (95% CI 0.86–1.79, p = 0.247), as detailed in Table 2.
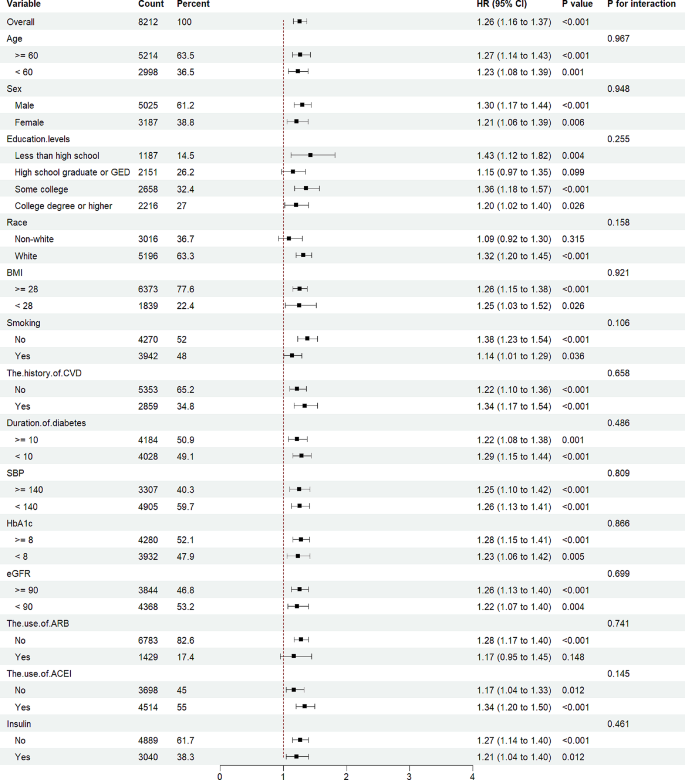
Interaction between subgroups to adverse renal outcomes
To explore the relationship between the TyG levels and adverse renal outcomes across various subgroups, interaction between subgroups to adverse renal outcomes was also evaluated. Analysis indicated that the p value for interaction among subgroups concerning worsening renal function was not statistically significant (p for interaction > 0.05), as depicted in Fig. 4. Further examination of the interactions relating to albuminuria and renal failure can be found in Supplemental Fig. 1 and Fig. 2, respectively. These findings suggested that the relationship between TyG levels and adverse renal outcomes remains consistent and significant across different subgroups of patients with T2DM, indicating a uniform impact of TyG levels on renal health irrespective of subgroup distinctions.
Discussion
Our investigation focused on the relationship between TyG index levels and adverse renal outcomes within a cohort of individuals with T2DM from the ACCORD study, also examining the consistency of these associations across different subgroups. We discovered a significant link between baseline TyG levels and the incidence of adverse renal outcomes, a relationship that persisted across various subgroups. Remarkably, this association remained evident even when accounting for established risk factors like serum creatinine levels, urinary protein content, and the use of ARB or ACEI. These findings highlighted the TyG index as a reliable predictor for early adverse renal outcomes among T2DM patients. Nonetheless, it’s critical to emphasize that the effectiveness of TyG in predicting late-stage renal adverse outcomes warrants further exploration. This study firstly utilized the ACCORD trial and underscored the TyG index’s potential as a tool in the early identification of T2DM patients in the United States at risk for renal complications, independent of traditional risk factors, suggesting its utility in clinical practice and future research directions.
Our study delved into the TyG index, it is very clear that the TyG index is an indicator composed of two risk factors, namely lipid-related and glucose-related factors, which reflect insulin resistance in the human body [21]. Previous research has described that fasting plasma glucose primarily reflects insulin resistance in the liver, while fasting triglycerides mainly reflect insulin resistance in adipocytes [9]. In fact, it was documented that the TyG index was the best index to identify individuals with insulin resistance, even superior to visceral adiposity indicators and other lipid parameters [7, 8, 22]. This index, first proposed in 2008, was shown to outperform the homeostasis model assessment-insulin resistance (HOMA-IR) index in identifying IR, demonstrating a sensitivity of 84.0% and specificity of 45.0% in a significant cross-sectional study of apparently healthy individuals [23]. Further research by Guerrero-Romero et al. in 2010 involving 99 participants with varying body weights and glucose tolerance highlighted the TyG index’s optimal performance for IR assessment, exhibiting high specificity (85.0%) and sensitivity (96.5%) compared to the gold standard, the Euglycemic-Hyperinsulinemia Clamp Test [22]. Since its inception, the TyG index has been established as a reliable and accessible tool for evaluating IR in individuals at high risk through extensive clinical studies. Previous research primarily focused on cardiovascular diseases (CVDs) such as coronary artery calcification (CAC), acute coronary syndrome (ACS), heart failure (HF), arterial stiffness (AS), stent restenosis, and stable coronary artery disease (CAD) [21]. Laura et al. utilized a large sample from the Vascular Metabolic CUN cohort (VMCUN cohort) over a median follow-up of 10 years to first suggest a positive association between the TyG index and CVD events, including, peripheral arterial disease (PAD), cerebrovascular disease and coronary heart disease (CHD), independent of confounding factors [24]. Despite the extensive application of the TyG index in cardiovascular research, limited studies have explored its relationship with adverse renal outcomes. Our current investigation assesses the TyG index as a potential risk factor for incident adverse renal outcomes in the T2DM population, with a focus on evaluating whether this significant association is consistent across different subgroups.
The relationship between triglyceride-glucose (TyG) index levels and chronic kidney disease (CKD) has been explored in some studies, revealing inconsistent results. Despite these discrepancies, a growing body of evidence suggested a strong link between TyG levels and adverse renal outcomes. A community-based cross-sectional study identified a significant association between higher TyG index levels and increased micro-albuminuria [25], while a cohort study in China reported that elevated TyG index levels were significantly correlated with a higher risk of developing albuminuria, particularly among individuals with metabolic dysfunction [26]. Further research has shown associations of TyG levels with acute kidney injury (AKI) [27], end-stage renal disease [28], hyperuricemia [29] and worsening renal function [30, 31]. These findings underscore the necessity for additional studies to clarify the relationship between the TyG index and CKD, especially in patients with T2DM who are at risk of insulin resistance. This area remains pivotal for ongoing research and clinical investigation.
This investigation aimed to assess the TyG index as a predictor for adverse renal outcomes among individuals with T2DM. Our findings robustly supported the TyG index as a valuable predictor for early renal adverse outcomes. After adjusting for confounding factors, the study revealed a significant association between TyG levels and key adverse renal outcomes, including worsening renal function and the onset of albuminuria. The UACR and eGFR, well-established markers for chronic kidney disease risk assessment, underscore the importance of regular monitoring to manage renal function in diabetic patients effectively. The potential lag in these markers becoming abnormal, indicating already present advanced kidney damage, highlights the urgent need for innovative biomarkers for early detection. Our results demonstrated TyG’s capability to effectively predict early renal adverse outcomes, addressing a critical gap in current biomarkers and facilitating timely interventions for individuals with elevated TyG levels. Notably, our study did not observe a significant link between TyG levels and the progression to renal failure, likely due to the constrained follow-up period which limited observing these specific outcome events. This absence of statistical significance underlines the necessity for extended research to further elucidate TyG’s relationship with late-stage renal adverse outcomes. Despite these considerations, our research offers compelling evidence of the TyG index as a reliable early indicator of adverse renal outcomes, presenting significant clinical utility.
The TyG index’s predictive capacity for cardiovascular diseases has been linked to several molecular mechanisms, including smooth muscle cell dysfunction, coagulation, endothelial dysfunction, metabolic flexibility [21]. However, the specific mechanisms connecting the TyG index to incident adverse renal outcomes are less defined, though several plausible explanations exist. Firstly, insulin resistance is known to correlate with elevated levels of inflammatory markers [32] and inflammation has been recognized as an independent risk factor for incident adverse renal outcomes. Secondly, IR may activate the mitochondrial electron transport chain, leading to the production of reactive oxidative stress (ROS), which in turn can cause kidney tissue fibrosis [15]. Thirdly, hyperinsulinemia, often associated with IR, can detrimentally impact renal function by promoting glomerular hyperfiltration, endothelial dysfunction, and increased vascular permeability [33]. Therefore, individuals with higher TyG index values are likely to experience more severe kidney function impairment and are at a higher risk for adverse renal outcomes.
The utilization of a large sample size, access to high-quality subject information, and the application of various statistical methods enable our study to identify independent associations between the TyG index and adverse renal outcomes, leading to robust conclusions. Nonetheless, it’s essential to recognize our study’s limitations. Even though we incorporate most recognized risk factors for adverse renal outcomes into our multivariable regression models, we cannot completely exclude the possibility of residual confounding factors. Moreover, it’s important to highlight that our findings are observational. Although they strongly indicate a correlation between TyG levels and adverse renal outcomes, future prospective intervention studies are crucial to definitively determine TyG levels’ causal effects on these outcomes. Additionally, due to a lack of fasting insulin levels in the ACCORD trial, we cannot compare whether TyG is better than HOMA-IR in predicting the occurrence of adverse renal outcomes. Finally, the population of the ACCORD trial included only high-risk patients with type 2 diabetes and additional studies are necessary to increase the generalizability of the results.
Conclusion
Our investigation focused on elucidating the relationship between the TyG index and adverse renal outcomes in individuals with T2DM. The outcomes of this study are significant, demonstrating a clear association between baseline TyG levels and adverse renal outcomes. Furthermore, our findings indicate that the TyG index could be a useful tool for risk stratification in predicting adverse renal outcomes among patients with T2DM. However, to enhance our understanding and validate these associations, further research is necessary. Future studies should aim to corroborate our results and explore whether interventions aimed at reducing TyG levels could offer benefits to patients with T2DM exhibiting elevated TyG levels. Such research has the potential to inform clinical practices, thereby improving the management and care of diabetic individuals at risk of developing adverse renal outcomes.
Data availability
No datasets were generated or analysed during the current study.
References
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14.
Dwyer JP, Parving HH, Hunsicker LG, Ravid M, Remuzzi G, Lewis JB. Renal dysfunction in the Presence of Normoalbuminuria in Type 2 diabetes: results from the DEMAND Study. Cardiorenal Med. 2012;2:1–10.
Umanath K, Lewis JB. Update on Diabetic Nephropathy: Core Curriculum 2018. Am J Kidney Dis. 2018;71:884–95.
Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–8.
Caro JF. Clinical review 26: insulin resistance in obese and nonobese man. J Clin Endocrinol Metab. 1991;73:691–5.
Khan SH, Sobia F, Niazi NK, Manzoor SM, Fazal N, Ahmad F. Metabolic clustering of risk factors: evaluation of triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr. 2018;10:74.
Cersosimo E, Solis-Herrera C, Trautmann ME, Malloy J, Triplitt CL. Assessment of pancreatic beta-cell function: review of methods and clinical applications. Curr Diabetes Rev. 2014;10:2–42.
Sanchez-Garcia A, Rodriguez-Gutierrez R, Mancillas-Adame L, Gonzalez-Nava V, Diaz Gonzalez-Colmenero A, Solis RC, Alvarez-Villalobos NA, Gonzalez-Gonzalez JG. Diagnostic Accuracy of the Triglyceride and Glucose Index for Insulin Resistance: A Systematic Review. Int J Endocrinol 2020, 2020:4678526.
Lopez-Jaramillo P, Gomez-Arbelaez D, Martinez-Bello D, Abat MEM, Alhabib KF, Avezum A, Barbarash O, Chifamba J, Diaz ML, Gulec S, et al. Association of the triglyceride glucose index as a measure of insulin resistance with mortality and cardiovascular disease in populations from five continents (PURE study): a prospective cohort study. Lancet Healthy Longev. 2023;4:e23–33.
Hong S, Han K, Park CY. The triglyceride glucose index is a simple and low-cost marker associated with atherosclerotic cardiovascular disease: a population-based study. BMC Med. 2020;18:361.
Yao Y, Wang B, Geng T, Chen J, Chen W, Li L. The association between TyG and all-cause/non-cardiovascular mortality in general patients with type 2 diabetes mellitus is modified by age: results from the cohort study of NHANES 1999–2018. Cardiovasc Diabetol. 2024;23:43.
Chiu H, Tsai HJ, Huang JC, Wu PY, Hsu WH, Lee MY, Chen SC. Associations between Triglyceride-Glucose Index and Micro- and Macro-angiopathies in Type 2 Diabetes Mellitus. Nutrients 2020, 12.
Yu Y, Meng Y, Liu J. Association between the triglyceride-glucose index and stroke in middle-aged and older non-diabetic population: a prospective cohort study. Nutr Metab Cardiovasc Dis. 2023;33:1684–92.
Xiong S, Chen Q, Zhang Z, Chen Y, Hou J, Cui C, Cheng L, Su H, Long Y, Yang S, et al. A synergistic effect of the triglyceride-glucose index and the residual SYNTAX score on the prediction of intermediate-term major adverse cardiac events in patients with type 2 diabetes mellitus undergoing percutaneous coronary intervention. Cardiovasc Diabetol. 2022;21:115.
Ren X, Jiang M, Han L, Zheng X. Association between triglyceride-glucose index and chronic kidney disease: a cohort study and meta-analysis. Nutr Metab Cardiovasc Dis. 2023;33:1121–8.
Chen N, Ma LL, Zhang Y, Chu X, Dong J, Yan YX. Association of long-term triglyceride-glucose index patterns with the incidence of chronic kidney disease among non-diabetic population: evidence from a functional community cohort. Cardiovasc Diabetol. 2024;23:7.
Kunutsor SK, Seidu S, Kurl S, Laukkanen JA. Baseline and usual triglyceride-glucose index and the risk of chronic kidney disease: a prospective cohort study. Geroscience; 2024.
Yu C, Shi Y, Wang T, Zhu L, Zhou W, Bao H, Cheng X. Triglyceride-glucose index change and chronic kidney disease progression in a Chinese hypertensive population. Front Endocrinol (Lausanne). 2024;15:1342408.
Pan Y, Zhong S, Zhou K, Tian Z, Chen F, Liu Z, Geng Z, Li S, Huang R, Wang H et al. Association between Diabetes Complications and the Triglyceride-Glucose Index in Hospitalized Patients with Type 2 Diabetes. J Diabetes Res : 2021, 2021:8757996.
Group AS, Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99:i21–33.
Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21:68.
Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, Jacques-Camarena O, Rodriguez-Moran M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–51.
Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304.
Sanchez-Inigo L, Navarro-Gonzalez D, Fernandez-Montero A, Pastrana-Delgado J, Martinez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46:189–97.
Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, Teliewubai J, Zhang Y, Xu Y. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc Diabetol. 2019;18:95.
Gao W, Wang J, Chen Y, Qiao H, Qian X, Xin Z, Zhao Z, Wang T, Xu Y, Xu M, et al. Discordance between the triglyceride glucose index and HOMA-IR in incident albuminuria: a cohort study from China. Lipids Health Dis. 2021;20:176.
Yang Z, Gong H, Kan F, Ji N. Association between the triglyceride glucose (TyG) index and the risk of acute kidney injury in critically ill patients with heart failure: analysis of the MIMIC-IV database. Cardiovasc Diabetol. 2023;22:232.
Fritz J, Brozek W, Concin H, Nagel G, Kerschbaum J, Lhotta K, Ulmer H, Zitt E. The triglyceride-glucose index and obesity-related risk of end-stage kidney disease in Austrian adults. JAMA Netw Open. 2021;4:e212612.
Li Q, Shao X, Zhou S, Cui Z, Liu H, Wang T, Fan X, Yu P. Triglyceride-glucose index is significantly associated with the risk of hyperuricemia in patients with diabetic kidney disease. Sci Rep. 2022;12:19988.
Lei L, Liang H, Qu Y, Zhong Q, Zhang Q, Dai L, Lu J, Xiao M, Zhao Z, Zhou F, et al. Association between triglyceride-glucose index and worsening renal function in the elderly. Front Nutr. 2022;9:951564.
Cui C, Liu L, Zhang T, Fang L, Mo Z, Qi Y, Zheng J, Wang Z, Xu H, Yan H, et al. Triglyceride-glucose index, renal function and cardiovascular disease: a national cohort study. Cardiovasc Diabetol. 2023;22:325.
Chen J, Wildman RP, Hamm LL, Muntner P, Reynolds K, Whelton PK, He J, Third National H, Nutrition Examination S. Association between inflammation and insulin resistance in U.S. nondiabetic adults: results from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27:2960–5.
Schrauben SJ, Jepson C, Hsu JY, Wilson FP, Zhang X, Lash JP, Robinson BM, Townsend RR, Chen J, Fogelfeld L, et al. Insulin resistance and chronic kidney disease progression, cardiovascular events, and death: findings from the chronic renal insufficiency cohort study. BMC Nephrol. 2019;20:60.
Acknowledgements
We are thankful for the contributions the ACCORD group made in data collection and sharing.
Funding
This project was supported by the Major Program of the National Natural Science Foundation of China (82090024).
Author information
Authors and Affiliations
Contributions
ZZP, JXP and PY contributed to study design. JXP, ZZP and PY contributed to data acquisition. PY, JXP and ZZP contributed to data analysis. PY, LH, JXP and ZZP contributed to drafting of the manuscript. LJT contributed to supervision and mentorship. The final version of the manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Ethics approval
The use of the ACCORD dataset in this study has been approved by the National Heart, Lung, and Blood Institute.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Figure S1:
Interaction between subgroups to albuminuria
Supplementary Material 2: Figure S2:
Interaction between subgroups to renal failure
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, P., Pu, J., Yuan, Q. et al. The prognostic value of triglyceride-glucose index to adverse renal outcomes in patients with type 2 diabetes mellitus: results from the cohort study of ACCORD. Diabetol Metab Syndr 16, 201 (2024). https://doi.org/10.1186/s13098-024-01439-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13098-024-01439-0